Introduction
Children with sickle cell anemia (SCA) are at increased risk of stroke as compared to age-matched peers (Ohene-Frempong et al, Blood, 1998). The Stroke Prevention Trial in SCA (STOP) demonstrated that with use of transcranial Doppler ultrasound (TCD), children at highest risk for stroke could be identified and started on chronic transfusion therapy, reducing stroke risk by > 90% (Adams et al, NEJM, 1998). This led to the adoption of the STOP protocol as standard of care. However, many sites use the transcranial Doppler imaging (TCDi) technique in place of the original non-imaging TCD technique used in STOP. Further, the NHLBI-funded DISPLACE (Dissemination and Implementation of Stroke Prevention in SCD) study demonstrated < 50% of children at participating sites had annual TCD screening (Kanter et al, JPHO, 2021). This sub-study used DISPLACE data to examine TCD report quality. We hypothesized there was high variability in TCD technique, unreliable reporting of variables, inconsistent definitions for diagnosing abnormal scans and that these inconsistencies would be worse with TCDi.
Methods
An IRB waiver was obtained. This sub-study used data manually extracted from TCD reports in the DISPLACE database. A computer-generated algorithm randomly selected 400 TCD/TCDi reports (200 of each type) from the 12,433 reports collected in DISPLACE for an alpha of 0.05. TCD type, patient age, vessels assessed, velocities measured, and diagnosis made (abnormal/normal) were extracted. Type of TCD was separately extracted from the DISPLACE database. We surveyed study site principal investigators regarding TCD methods used in the study period (2012 - 2016).
Results
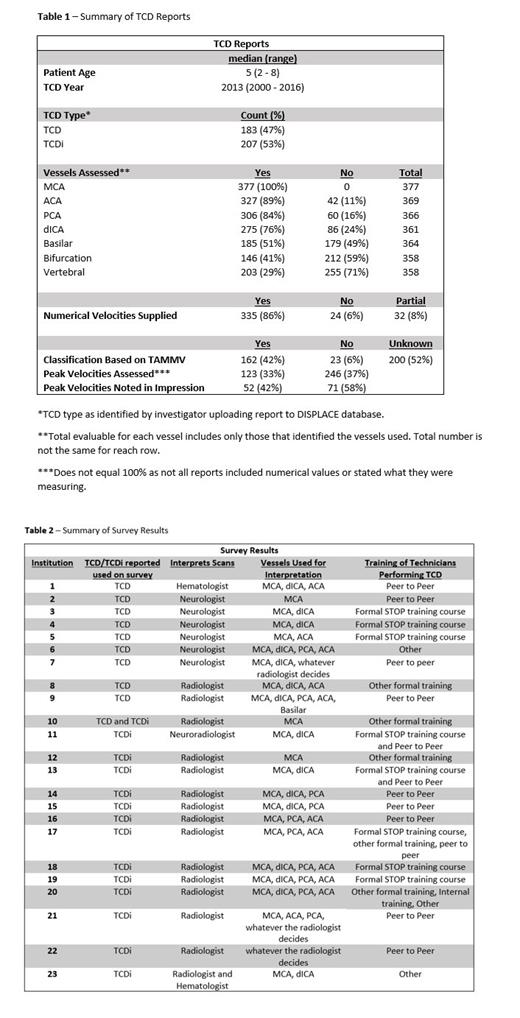
Three hundred and ninety-one TCD/TCDi reports were reviewed from 26 institutions. The median patient age at time of TCD was 5 years (2 - 8 years) and the median year of TCD completion was 2013 (2000 - 2016).
On initial review of the TCD/TCDi reports, there was significant variability in how results were classified as “abnormal”, and reports were often inconsistent with STOP definitions of normal/abnormal. Thus, we focused on the quality and completeness of reports. Table 1 details the information included in the reports. Notable findings:
• Use of TCDi was not specifically stated in 96% of TCDi reports.
• 52% did not clearly identify the time averaged mean maximum velocity (TAMMV) (the key variable required per STOP criteria).
• 30% of reports did not assess/did not report assessment of the distal internal carotid artery (dICA).
• 14 reports (3.6%) did not include any information about which vessels were assessed.
• Only 42% of scans clearly stated that classification of normal vs. abnormal was based on the correct measure (TAMMV).
Surveys sent to each site to assess TCD practices from 2012-2016 were evaluated (Table 2). There was substantial variability regarding which vessels were examined for both TCD and TCDi, ranging from 1 - 5 vessels. The type of provider interpreting scans varied and only 8 institutions (35%) had ultrasound technicians who underwent formal STOP training.
Conclusion
TCD results have critical implications for medical decision-making in SCA, but our study reveals a lack of quality assurance and consistency with sickle cell stroke risk screening using TCD/TCDi. Notably, many reports did not identify which vessels were used to assess stroke risk, did not report on the correct velocity measurement, neglected to measure or report the TAMMV, or made diagnoses based on the wrong measurements or wrong vessels. Additionally, a large percentage of reports did not note whether a TCD or TCDi was performed. Surveys from 23 DISPLACE sites showed significant variation in the method of training for those performing TCDs.
With this extreme variation, we recommend creation of a standardized template for TCD reports to be utilized across institutions. We also recommend those interpreting the scans gain expertise in reading and evaluating TCD/TCDi specifically for people with SCA. National quality improvement studies are necessary to implement these recommendations and inform further refinements, especially in the use of TCDi, and assessment of vessels beyond the middle cerebral artery and dICA. This requires engagement of stakeholders, identification of barriers to implementation, and funding. With these interventions, we continue to work towards meeting the recommended screening and interventions instituted by the NHLBI over 25 years ago.
Disclosures
Hulbert:Bluebird Bio: Consultancy; NovoNordisk: Research Funding. Hsu:Aruvant: Other: Data Safety Board Member ; Asklepion Pharmaceuticals: Research Funding; Hilton Publishing, Emmaus, Fresnius: Consultancy. Bhasin:Novartis, GBT/Pfizer, Forma: Honoraria, Research Funding. Adams:Duke University, Univ of Miami, Global Blood Therapeutics, Pfizer, Novo-Nordisk: Consultancy; Zeriscope, Inc: Other: Co-founder . Kanter:Bausch: Consultancy; ECOR-1: Consultancy; Fulcurm: Consultancy; Chiesi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Watkins, Lourie, Roll & Chance: Consultancy; Austin Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Guidepoint Global: Honoraria; Vertex: Consultancy; NHLBI: Research Funding; CDC: Research Funding; HRSA: Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; BEAM: Consultancy, Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; National Alliance of Sickle Cell Centers: Other: President.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal